“Do not submit extra labels”
This error occurred if you submitted more than one label in the Principal Display Panel section. Each label should have its own Principal Display Panel section and you only need one label per strength even if there are multiple labels.
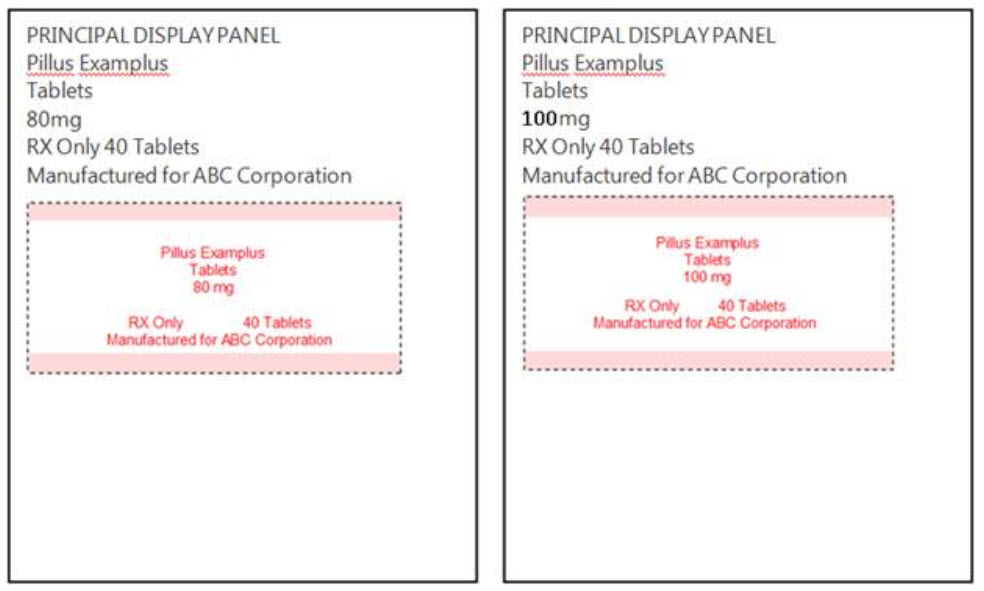
For example, if you have a pill that has 100mg of active ingredient that comes in bottles of 20, 40, and 60 then you only need to provide one label. Only provide two labels if there are multiple strengths such as the example below of Pillus Examples that comes in 80mg and 100mg strengths with multiple packaging options.
Comments
0 comments
Please sign in to leave a comment.